|
|
|
|
|
|

|
 Clopidogrel Bisulfate
Clopidogrel Bisulfate |
|
 Clopidogrel Bisulfate
Clopidogrel Bisulfate |
|
|
|
|
|
HOME >>
API >>
API List1
>>
Clopidogrel Bisulfate

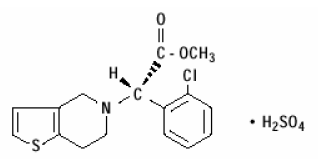
structural formula is as follows:

DRUG DESCRIPTION
(clopidogrel bisulfate) is a thienopyridine class inhibitor of P2Y12 ADP
platelet receptors. Chemically it is methyl (+)- (S)-α-
(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5 (4H)-acetate sulfate
(1:1). The empirical formula of clopidogrel bisulfate is C16H16ClNO2S•H2SO4
and its molecular weight is 419.9.
|
The structural formula is as follows:
|
 |
|
Clopidogrel Bisulfate |
Clopidogrel bisulfate is a white to off-white
powder. It is practically insoluble in water at neutral pH but freely
soluble at pH 1. It also dissolves freely in methanol, dissolves sparingly
in methylene chloride, and is practically insoluble in ethyl ether. It has a
specific optical rotation of about +56°.
Plavix for oral administration is provided as either pink, round, biconvex,
debossed, film-coated tablets containing 97.875 mg of clopidogrel bisulfate
which is the molar equivalent of 75 mg of clopidogrel base or pink, oblong,
debossed film-coated tablets containing 391.5 mg of clopidogrel bisulfate
which is the molar equivalent of 300 mg of clopidogrel base.
Each tablet contains hydrogenated castor oil, hydroxypropylcellulose,
mannitol, microcrystalline cellulose and polyethylene glycol 6000 as
inactive ingredients. The pink film coating contains ferric oxide,
hypromellose 2910, lactose monohydrate, titanium dioxide and triacetin. The
tablets are polished with Carnauba wax.
INDICATIONS
Acute Coronary Syndrome (ACS)
For patients with non-ST-segment elevation ACS [unstable angina (UA)/non-ST-elevation
myocardial infarction (NSTEMI)], including patients who are to be managed
medically and those who are to be managed with coronary revascularization,
Plavix has been shown to decrease the rate of a combined endpoint of
cardiovascular death, myocardial infarction (MI), or stroke as well as the
rate of a combined endpoint of cardiovascular death, MI, stroke, or
refractory ischemia.
For patients with ST-elevation myocardial infarction (STEMI), Plavix has
been shown to reduce the rate of death from any cause and the rate of a
combined endpoint of death, re-infarction, or stroke. The benefit for
patients who undergo primary percutaneous coronary intervention is unknown.
The optimal duration of Plavix therapy in ACS is unknown. Recent MI, Recent
Stroke, or Established Peripheral Arterial Disease
For patients with a history of recent myocardial infarction (MI), recent
stroke, or established peripheral arterial disease, Plavix has been shown to
reduce the rate of a combined endpoint of new ischemic stroke (fatal or
not), new MI (fatal or not), and other vascular death.
WARNINGS
Thrombotic thrombocytopenic purpura (TTP)
TTP has been reported rarely following use of clopidogrel bisulfate,
sometimes after a short exposure (<2 weeks). TTP is a serious condition that
can be fatal and requires urgent treatment including plasmapheresis (plasma
exchange). It is characterized by thrombocytopenia, microangiopathic
hemolytic anemia (schistocytes [fragmented RBCs] seen on peripheral smear),
neurological findings, renal dysfunction, and fever. (See ADVERSE
REACTIONS.)
PRECAUTIONS
General
Clopidogrel bisulfate prolongs the bleeding time and therefore should be
used with caution in patients who may be at risk of increased bleeding from
trauma, surgery, or other pathological conditions (particularly
gastrointestinal and intraocular). If a patient is to undergo elective
surgery and an antiplatelet effect is not desired, clopidogrel bisulfate
should be discontinued 5 days prior to surgery.
Due to the risk of bleeding and undesirable hematological effects, blood
cell count determination and/or other appropriate testing should be promptly
considered, whenever such suspected clinical symptoms arise during the
course of treatment (see ADVERSE REACTIONS).
In patients with recent TIA or stroke who are at high risk for recurrent
ischemic events, the combination of aspirin and clopidogrel bisulfate has
not been shown to be more effective than clopidogrel bisulfate alone, but
the combination has been shown to increase major bleeding.
GI Bleeding
In CAPRIE, clopidogrel bisulfate was associated with a rate of
gastrointestinal bleeding of 2%, vs. 2.7% on aspirin. In CURE, the incidence
of major gastrointestinal bleeding was 1.3% vs 0.7% (clopidogrel bisulfate +
aspirin vs. placebo + aspirin, respectively). Clopidogrel bisulfate should
be used with caution in patients who have lesions with a propensity to bleed
(such as ulcers). Drugs that might induce such lesions should be used with
caution in patients taking clopidogrel bisulfate.
Use in Hepatically Impaired Patients
Experience is limited in patients with severe hepatic disease, who may have
bleeding diatheses. Clopidogrel bisulfate should be used with caution in
this population.
Use in Renally Impaired Patients
Experience is limited in patients with severe renal impairment. Clopidogrel
bisulfate should be used with caution in this population.
Information for Patients
Patients should be told that it may take them longer than usual to stop
bleeding, that they may bruise and/or bleed more easily when they take
clopidogrel bisulfate or clopidogrel bisulfate combined with aspirin, and
that they should report any unusual bleeding to their physician. Patients
should inform physicians and dentists that they are taking clopidogrel
bisulfate and/or any other product known to affect bleeding before any
surgery is scheduled and before any new drug is taken.
Pregnancy
Pregnancy Category B. Reproduction studies performed in rats and rabbits at
doses up to 500 and 300 mg/kg/day (respectively, 65 and 78 times the
recommended daily human dose on a mg/m2 basis), revealed no evidence of
impaired fertility or fetotoxicity due to clopidogrel. There are, however,
no adequate and well-controlled studies in pregnant women. Because animal
reproduction studies are not always predictive of a human response,
clopidogrel bisulfate should be used during pregnancy only if clearly
needed.
Nursing Mothers
Studies in rats have shown that clopidogrel and/or its metabolites are
excreted in the milk. It is not known whether this drug is excreted in human
milk. Because many drugs are excreted in human milk and because of the
potential for serious adverse reactions in nursing infants, a decision
should be made whether to discontinue nursing or to discontinue the drug,
taking into account the importance of the drug to the nursing woman.
Pediatric Use
Safety and effectiveness in the pediatric population have not been
established.
Geriatric Use
Of the total number of subjects in controlled clinical studies,
approximately 50% of patients treated with clopidogrel bisulfate were 65
years of age and over. Approximately 16% of patients treated with
clopidogrel bisulfate were 75 years of age and over.
The observed difference in risk of thrombotic events with clopidogrel plus
aspirin versus placebo plus aspirin by age category is provided in Figure 3
(see CLINICAL STUDIES). The observed difference in risk of bleeding events
with clopidogrel plus aspirin versus placebo plus aspirin by age category is
provided in Table 3 (see ADVERSE REACTIONS).
DOSAGE AND ADMINISTRATION
Recent MI, Recent Stroke or Established Peripheral Arterial Disease
The recommended daily dose of clopidogrel tablets is 75 mg once daily.
Acute Coronary Syndrome
For patients with acute coronary syndrome (unstable angina/non-Q-wave MI),
clopidogrel bisulfate should be initiated with a single 300 mg loading dose
and then continued at 75 mg once daily. Aspirin (75 mg-325 mg once daily)
should be initiated and continued in combination with clopidogrel bisulfate.
In CURE, most patients with Acute Coronary Syndrome also received heparin
acutely (see CLINICAL STUDIES).
Clopidogrel tablets can be administered with or without food.
No dosage adjustment is necessary for elderly patients or patients with
renal disease (See Clinical Pharmacology: Special Populations.)
HOW SUPPLIED
Clopidogrel Tablets USP, 75 mg are reddish-brown, round, unscored, film
coated tablets, imprinted "APO" on one side and "CL" over "75" on the other
side. They are supplied as follows:
Bottles of 30 NDC 60505-0253-1
Bottles of 90 NDC 60505-0253-2
Bottles of 1000 NDC 60505-0253-3
100 Unit Dose NDC 60505-0253-4
Storage
Store at 25° C (77° F); excursions permitted to 15° to 30° C (59° to 86° F)
[See USP Controlled Room Temperature].
 
|














